Metal Mouth—a Condition Affecting Cancer Patients and a Prospective Treatment with Lactoferrin

By Morgan Shock, Science Communicator, Water INTERface IGEP
The National Cancer Institute states that about 39.5% of people will be diagnosed with a form of cancer at some point in their life. Cancer and its treatment affect patient quality of life in many ways. Beyond the most visually obvious and well-known effects such as hair loss, there are so many more that don’t have nearly as much public recognition. One of these lesser-known impacts is Metal Mouth. This is a condition in which patients experience metallic flavors or aftertastes when eating or drinking or even just generally in their mouth without doing anything at all. This might not seem like a big deal when we are talking about cancer, but small things can have big consequences. Turns out, most people don’t enjoy all their meals tasting funky. This makes eating (a thing you need to do in order to, you know, live) into an unpleasant chore at a time when life is already in such a delicate balance. While it is never a good time to develop disordered eating or be malnourished, during cancer treatment is especially unfortunate timing. Thankfully, researchers at Virginia Tech and Wake Forest School of Medicine have been collaborating in order to understand the causes of Metal Mouth and investigate potential treatment. This research wouldn’t be possible without the Water INTERface Interdisciplinary Graduate Education Program (IGEP) at Virginia Tech which focuses on the interaction between water and health. This article is going to give you the background you need in order to understand what they have figured out so far.
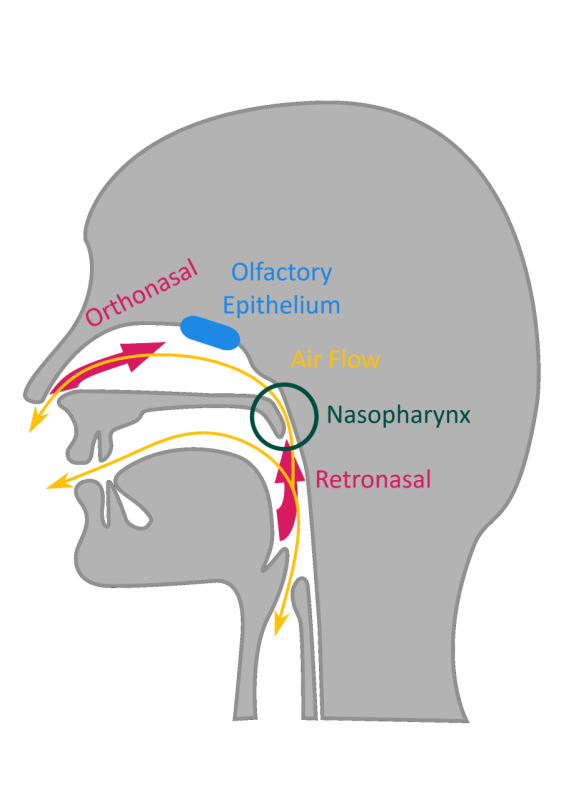
So to get started, what causes Metal Mouth? Well, to get into that we first need to understand how taste works. Here is a cartoon illustrating the relevant facial cavities to smelling and tasting. Yes, I did just slide smell on in there when I said we were going to talk about taste. As anyone who has ever had a stuffed-up nose can tell you: without smell, taste loses a lot of oomph. So, once we go over tasting we will come back to smelling and how these two senses combine into the experience called flavor.
Without smell, there are really only five tastes: Sweet, Salty, Sour, Savory and Bitter. To get more nuanced than that you have to involve smelling and at that point we stop calling the experiences tastes and start calling them flavors. For the most part, Metal Mouth is a flavor more so than a taste. Many patients report a bitter taste to be part of the experience, and some report sourness. Often part of bitter taste is the accompanying drying feeling called astringency. Now let’s dive into smelling and how important it is the whole metal mouth experience.
In the cartoon, you can see that mouth and nose both open into a space that is connected at the back of the throat. This means that you can breathe through either your mouth or your nose and that air can flow around in this space while you chew and swallow, as indicated by the gold arrows. Air flow is critical to your sense of smell because it depends on air reaching a particular spot toward the top and back of the nasal cavity, shown here as a blue pill-shape. This spot has millions of nerve endings that are ready to interact with odor molecules in the air. The proper name of this spot is the olfactory epithelium, which effectively means “smell-related skin-like tissue.” Looking at the cartoon again, you can see that air can flow by this area from two different directions. When air with smells comes in through the nose, it is technically called orthonasal odor. Ortho- is a bit of a loaded prefix here because it means “straight, right, proper.” It would be more productive to think of this as front-smelling. The opposite of front-smelling would then logically be back-smelling, which is exactly what retronasal odor is. As you swallow, the smelly air is pulled from the mouth and out the nose to get it to flow past the relevant area.
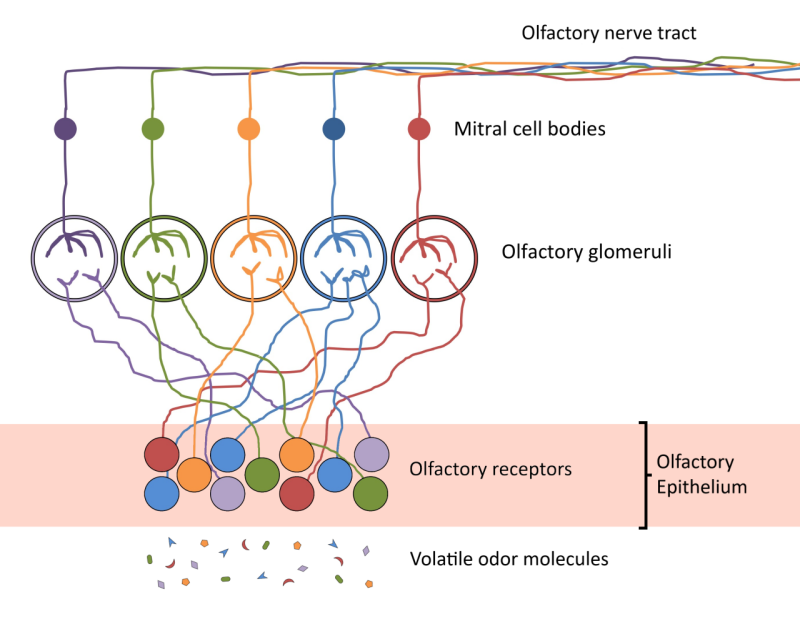
So, what in the air is it that we are actually smelling and how do we tell them all apart? I mentioned odor molecules earlier and another term for that you might have heard before is volatiles. Volatiles are molecules that easily evaporate at low temperatures and can thus be floating around in the air. All of the nerve endings in the olfactory epithelium have receptors that interact with various odor molecules. Humans have about 350 different receptor types, but we evolved a really cool system to be able to use them to tell apart way more smells than that, literally millions of them. In the olfactory epithelium, odor molecules interact with a few types of receptors, then each type of receptor sends signals to a receiver (called an olfactory glomerulus) that forwards it to the brain (see next cartoon). Your brain “sees” that pattern and identifies the associated smell. This information can then be combined with what’s coming from your taste buds on your tongue in a way that your brain combines taste and odor into the experience of flavor.
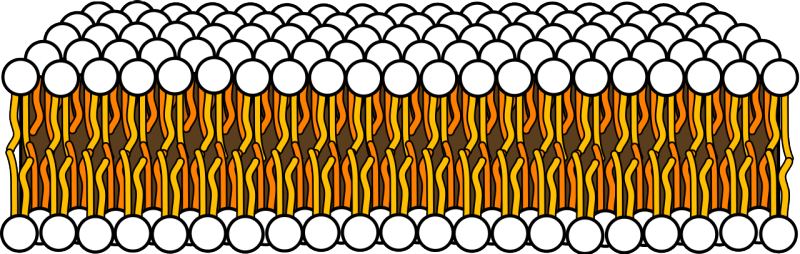
Here is where I throw you a curve ball: while you do taste metals like iron or copper, what you smell that then adds up to metallic flavor isn’t the metal at all, but odorous volatiles. For this to make sense, we need to get into some chemistry. If you aren’t as familiar as you’d like to be with the basics, check out the “Soooo, Chemistry…” section at the bottom of the article. When metal ions, like copper and iron, are in your mouth, they dissolve in your saliva and interact with living mouth tissues. When they react with certain taste receptors, you taste sour or bitter. But that’s far from the only reaction happening. Metal ions can react with the molecules in the cells that make up the lining of your mouth itself! The outer surfaces of your cells are made of a fatty membrane, the technical name being lipid bilayer, that controls how water and other materials move between the inside and outside of your cells. The lipids have long chains of carbon atoms, also called a fatty acid. The metal ions and oxygen in your breath react with the lipids, causing chunks to break off the fatty acid chain forming new types of molecules called aldehydes and ketones. These new molecules are odorous volatiles that can then move retronasally with your breath out through your nose, interacting with receptors in your olfactory epithelium on the way. The metallic smell/flavor you know is actually a byproduct of the lipid lining of your mouth being attacked by metals!
So why do cancer patients experience so much more metal flavor than other people? To be honest, we aren’t sure just yet. We know that cancer patients whose tumors are in the neck or head area are more likely to experience changes to their senses of smell and taste than those whose tumors are other places. We know that for the most part these problems don’t seem to be caused by cancer itself, but by the therapies used to treat it. Cancer is difficult to treat because it is our own bodies growing in such a way that it is harmful and trying to tell the difference between the harmful growth and everything else can be complicated. Most cancer treatments target cells that are growing and dividing quickly, but there are healthy cells in our bodies that do that under normal circumstances too. Taste buds, olfactory receptor cells, hair follicles, and skin cells both those on your actual skin and the ones lining your digestive system are also hit by these treatments. With radiation, we can limit this spillover damage by being careful and precise with application, but sometimes things are close enough that some spillover is unavoidable. There are several places that, if damaged, could change our senses of taste and smell. Oncologist Dr. Glenn Lesser and his colleagues at the Wake Forest School of Medicine tracked how much radiation was hitting these various spaces while treating patients with brain tumors. They found that changes to taste and smell seemed to be associated with high levels of radiation hitting the nasopharynx, the back part of your throat up behind your nose which is circled in dark green on the first cartoon, and with tumors located in a particular part of the brain called the temporal lobe. “We found some interesting possible connections, but the study population was small so we just can’t be certain yet,” said Dr. Lesser. “We need to keep working with patients until we have a better idea of what is going on.” There is also evidence that chemotherapy changes saliva composition. The exact mechanism hasn’t been pinned down yet, but cancer patients who have received chemotherapy and reported changes to smell and taste, including metal mouth, tend to have more dissolved iron in their saliva.
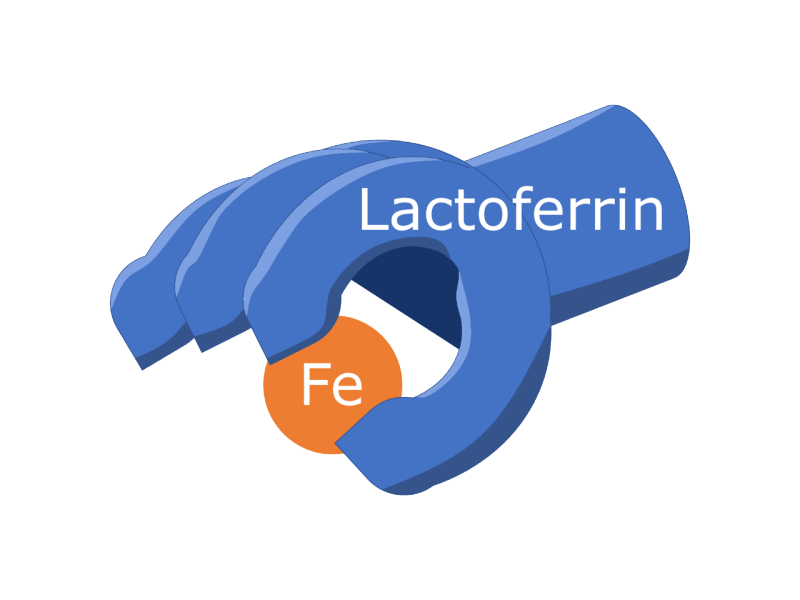
Even without knowing exactly what ultimately causes metal mouth, we can still do something about all that excess iron. The important point is that metal ions are reactive and breakdown lipids in the mouth to form odorous volatiles. So, if we want to stop this mayhem, and we do because eating and metal flavors don’t tend to mix well, a possible option is to prevent the whole process from even getting started by controlling the metal ions. Chelating agents bind metal ions (chelate is from the Greek for “claw” because chelators grab up metal ions). One group of chelators is the transferrins, which are proteins so named for their role in transporting iron ions. Lactoferrin is a transferrin that is especially common in human milk and present in human saliva, as well as in other mammals. “We tested the effectiveness of lactoferrin in alleviating metallic flavor and were surprised by how quickly and effectively it eliminated the metallic flavor of iron in the human mouth” said Dr. Andrea Dietrich, faculty member with the Virginia Tech Water INTERface IGEP and Department of Civil and Environmental Engineering. Dr. Dietrich and her colleagues figured this out by having volunteers drink some dissolved iron and the various treatments: plain water, as a control; the chelating agent lactoferrin which is more finely tuned to iron, and ethylenediaminetetraacetic acid (EDTA), a multipurpose chelating agent found in consumer products. EDTA was slightly better than water, but not by much. But lactoferrin? Lactoferrin completely removed metallic flavor across the board.
So, lactoferrin works for people simulating Metal Mouth, but what about actual cancer patients? And in a form that’s more readily available? I mentioned earlier that some lactoferrin is already present in human saliva. That’s because it plays an important role in regulating a lot of different processes in a healthy body. Lactoferrin supplements are available at many pharmacy or grocery stores. When cancer patients with Metal Mouth took 250 mg lactoferrin supplements three times a day for a month, they experienced less trouble with Metal Mouth. Even after another month without taking lactoferrin, the improvements not only persisted, but Metal Mouth troubles were even lower. This study, another collaboration between Virginia Tech and Wake Forest School of Medicine, also showed that while these cancer patients started off with a lot more iron in their saliva than healthy people, after taking the lactoferrin supplements their salivary iron levels were much closer to normal, even a month later. “We believe that ingesting lactoferrin supplements makes more lactoferrin available in the saliva to bind iron. This prevents the lipid-oxidation reactions that make odorous volatiles that contribute to metal mouth”, said Dr. Susan Duncan, faculty member with the Virginia Tech Water INTERface IGEP and the Department of Food Science and Technology.
Through collaboration, these interdisciplinary researchers identified a problem, learned more about its causes, and found a promising solution. Metal mouth is a serious quality of life issue for cancer patients that can greatly affect their health in an already stressful time, but lactoferrin could be a solution, helping patients have a better chance for finding comfort and getting enough nutrition. This is only one example of the benefits of the multidisciplinary approach encouraged by the Virginia Tech Water INTERface IGEP.
Soooo, Chemistry…
One of the first things you learn in beginner’s chemistry is this picture:
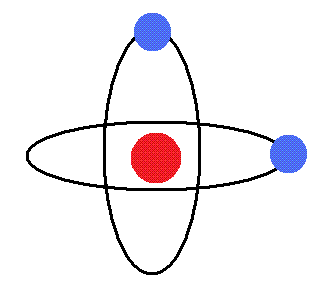
An Atom. It comes with a relatively dense bit in the middle called the nucleus (the red circle) made of smaller protons and neutrons and a lot of space around that in which even smaller electrons (the blue circles) are zooming around. The elements that easily let go of their electrons are metal atoms. When metals give up electrons, they become ions with positive charges. The electrons released by metals can be captured by molecules to cause chemical reactions. Keeping track of what electrons are up to is the foundation of chemistry. Chemically, something is a metal when a bunch of nuclei (the plural of nucleus because Latin) effectively pool all their loose electrons into something like a soup with the electrons being the broth and the nuclei being the floaty solids like noodles or tiny pieces of chicken and vegetables. But those elements can do other things as well, especially when non-metals are invited to the party. When metals and non-metals get together, the metals stop acting like a soup because their loose electrons get snatched up by the non-metals. Then each metal atom is stuck to the non-metal atom that took its electron(s) in what is called an ionic bond and together they are called a salt. If they separate, the non-metal still has the metal’s electron(s), so the non-metal has some negative charge, and the metal has some positive charge. Things with charges like this are called ions, which is where we got the term ionic bond (a bond between ions). These bonds are weak enough that salts dissolve in water. Other bonds, called covalent bonds, are a bit stronger. Metals can make those too, and more importantly here, they can participate in reactions that break such bonds in other molecules.
The “Metal Mouth” narrative is a successful, multi-year, collaborative project that incorporates contributions from these Virginia Tech Graduate Students.
- Ms. Kerri (Martin) Hamilton, currently at the US Department of Agriculture
- Dr. JaeHee Hong, currently a professor at Seoul National University
- Dr. Susan Mirlohi, currently a professor at California State University-Fresno
- Dr. Pinar Ömür-Özbek, currently a professor at Colorado State University
- Dr. Aili Wang, currently a professor at Chengdu University


