Effect of water minerals on taste perception and emotional response of sweetened beverage
Dr. Duncan and her student Aili (graduate of Water INTERface IGEP) have published a research on Food Quality and Preference recently, which investigated the taste interaction between sweet and metallic flavor for the first time. This study showed that sweetness of commercial sweeteners varied with different concentrations of water minerals such as Fe2+, Ca2+, Mg2+ and Na+. Since minerals (Fe2+, Ca2+, Mg2+ ,Na+, K+ , Cl–) at certain concentration (moderate-hard water) can amplify the sweet perception through taste interaction, the observations in this study may be applied to reduce the use (cost) of sweeteners in production. It is interesting to find that high concentration of iron and water hardness significantly increased (p < 0.05) the sweetness of sucrose, honey and ace-K. Sweet-metallic taste interaction between sucrose and ferrous ions significantly (p < 0.05) increased the acceptance of very hard water (3 mg Fe/L). According to the relatively high acceptability and positive emotional response, sucrose may serve as the sweetener in beverage products containing bitter/metallic metal ions. Although not proven in acceptance test, ace-K, saccharin, and sucralose may be suitable calorie-free sweeteners for beverage with bitter/metallic metal ions.
Results of this study will provide guidance for beverage industry to choose the appropriate sweeteners for their products to maximize the sweetness with less cost. At the same time, this study also helps beverage processors to establish criteria for quality of water used in beverage products. Sweet-metallic taste interaction may extend its application to other food products containing bitter/metallic constituents, such as polyphenol, tannin, glycosides, some amino acids and metal ions. Its potential in amplifying sweetness and increasing acceptability may be able to mask inherent metallic taste of functional food/beverage, supplements or medicine that are fortified by health beneficial components but with poor taste quality.
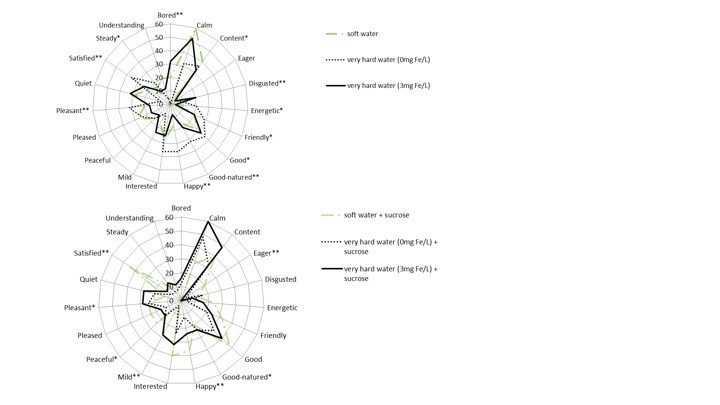
*Abstract:Although sweeteners are widely used additives in beverages, taste interaction between sweeteners and minerals in water is rarely reported. The objective was to investigate the influence of different concentrations of iron and water hardness on taste perception of sweetened beverages and characterize the corresponding emotional profiles. Taste interaction was developed by dissolving five natural and artificial sweeteners [sucrose, honey, sucralose, saccharin, and acesulfame potassium (ace-K)] into four synthetic waters [soft water (0 mg Fe/L), moderate hard water (0.3 mg Fe/L), hard water (1 mg Fe/L) and very hard water (3 mg Fe/L)], respectively. Sweet and metallic taste intensity of different combinations were compared by pairwise ranking tests. Acceptability and emotional response on sucrose sweetened beverage with and without the addition of iron was evaluated by 9-point hedonic score test and check-all-thatapply emotional term ballot. Iron (Fe2+) created metallic flavor in drinking water and produced bored and disgusted feelings for consumers. Other minerals such as Ca2+, Mg2+ and Na+ at subthresholds impacted taste perception of water. Sweetness of sweeteners was varied with different concentrations of minerals in water and with different types of sweeteners. High concentration of iron and water hardness significantly increased (p < 0.05) the sweetness of sucrose, honey and ace-K. Sweet-metallic taste interaction between sucrose and ferrous ions significantly (p < 0.05) increased the acceptance of very hard water (3 mg Fe/L), and created a unique emotional profile- ‘‘mild”. Distribution of emotional profiles could be different between samples with the same hedonic scores and provide more in-depth information than acceptance test.
* Wang, Aili, Susan E. Duncan, and Andrea M. Dietrich. 2016. Effect of iron on taste perception and emotional response of sweetened beverage under different water conditions. Food Quality and Preference. 54: 58-66.